Arrange the Following Elements in Order of Increasing Metallic Character
Hence the order of increasing metallic character is P Si Be Mg Na. 6- least Ba Select.
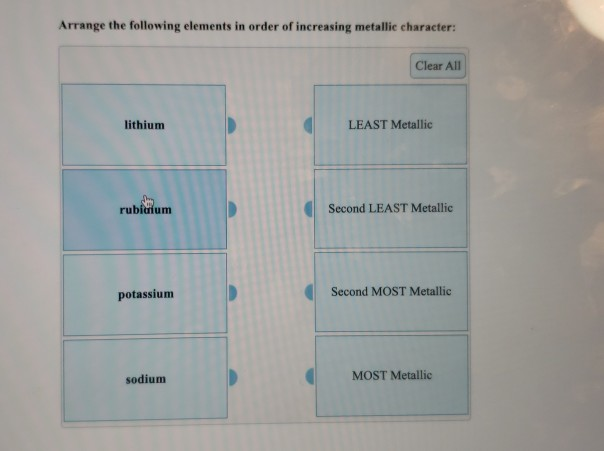
Solved Arrange The Following Elements In Order Of Increasing Chegg Com
Si Be Mg Na P.

. Arrange the following elements in order of decreasing metallic character. Re Co Db Ga W. In the periodic table of elements the metallic character decreases from left to right and it increases down a column.
LiCorrect answer is option B. A Carbon C b Silicon Si c Boron B d Fluorine F 3. A Covalent bond b Ionic bond c Hydrogen bond d Metallic bond 2.
Si Be Mg Na P. Arrange the following elements in the increasing order of metallic character. Arrange the following elements in increasing order of their atomic radii Li Be F N Asked on 29th Jan 2021 By considering their position in the Periodic Table which one of the following elements would you expect to have maximum metallic characteristicsGa Ge.
Problem 78 Easy Difficulty. Give reason for your answer. Tc Hf Ba Nb Cd-3.
Metallic character increases down a group and decreases along a period as we move from left to right. Answer According to Periodic properties Metallic character increases from top to bottom in groups and decreases from left to right in periods. Clear All boron LEAST Metallic beryllium Second LEAST Metallic carbon Second MOST Metallic nitrogen MOST Metallic Arrange the following elements in order of increasing metallic character.
Understand why the trends. Arrange the following elements in order of increasing metallic character. Strontium has the most metallic character because it is below and to the left of the remaining elements.
S Se Sb In Ba Fr. Arrange the following elements in order of increasing metallic character. Among K Mg and Ca K potassium will be more metallic because it is an alkali metal.
Nitrogen antimony Z51 arsenic Z33 phosphorus. Thus arranging the given elements according to increasing metallic order we have. Arrange the following elements in increasing order of metallic character potassium calcium gallium Germanium - 1743659.
Ti Ra Sr Fr Mn 2. And metallic character trends on the periodic table. Eqrm F- Na eq and eqrm N3- eq.
Arrange the following elements in order of their decreasing metallic character. Gallium has the second most metallic character because it is located below aluminum in the same column and below and to the left of the remaining elements. Ti Ra Sr Fr Mn-2.
Tc Hf Ba Nb Cd-3. Arrange the following in order of increasing ionic radius. Write your answers in your notebookon a separate sheet of paper.
Non metallic character decreses down the groupnon metallic character increase from left to righthence aswr B Arrange the following elements in the order of their increasing non-metallic characterLi O C Be FaF. Arrange the following elements in order of increasing metallic character. Mg P Si Na Be.
Li Be F N. Arrange the following elements in increasing order of metallic character Si Be Mg Na P. Clear All cesium LEAST Metallic sodium Second LEAST Metallic potassium Second MOST Metallic.
S Se Sb In Ba Fr. Arrange the following elements in the increasing order of their. Sr Ga Al Si P N.
Arrange the following elements in order of increasing metallic character. Arrange the following elements in order of increasing metallic character. Na Si Cl Mg Al - Science and Technology 1.
Arrange the following elements in the increasing order of their metallic character. Arrange the following elements Li B O Be NF in the order of metallic character. 100 17 ratings In periodic table m from left to right in any period meta.
Re Co Db Ga W-. So the overall order will be. Ge Ga Mg Ca K.
Sc Fe Rb Br O Ca F Te. Identify the element in a 2nd period and 14th group with valency 4. So the correct answer is Option C.
Fr Sn In Ba Se. Calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes in period 3 of group 2. Electronic configuration of an element x is 281 and for Y 287 then mention the bond existing between 2 elements.
Arrange the following in the increasing order of their metallic character. View the full answer. I atomic radii ii non metallic character.

Arrange The Following Elements In Increasing Order Of Metallic Character Na Mg Al K Brainly In
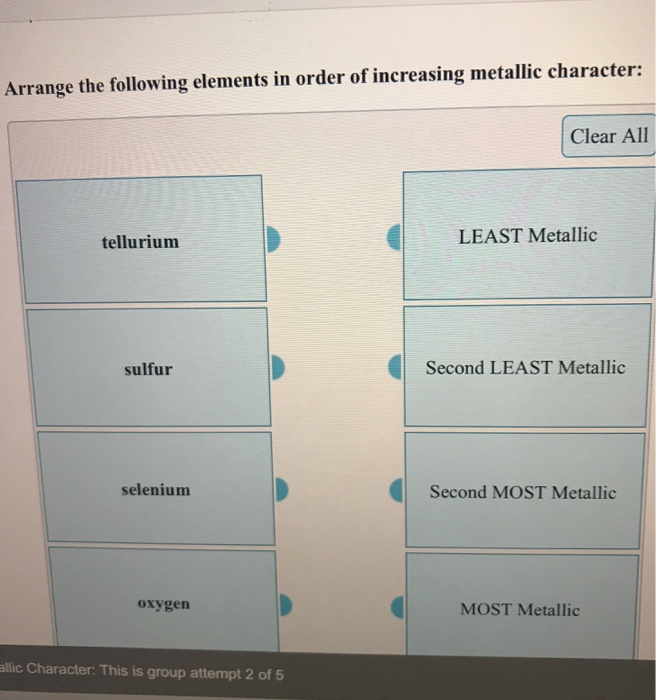
Solved Arrange The Following Elements In Order Of Increasing Chegg Com

How Do You Arrange Elements In Order Of Increasing Metallic Character
No comments for "Arrange the Following Elements in Order of Increasing Metallic Character"
Post a Comment